Covid Vaccine
November 9, 2020
Operation Warp Speed is what the US Government is calling its COVID-19 vaccine approval and distribution effort. In record speed, the COVID-19 vaccine, slated to release to a specific demographic, is said to be available in late November.
This past week, AstraZeneca and Johnson & Johnson, two companies in the late stages of COVID vaccine approval whose trials were halted, returned to research after reporting neurological developments caused by the vaccine.
Pfizer, Johnson and Johnson, Moderna, AstraZeneca, and BioNTech have reached the late stages of FDA vaccine requirements. Pfizer’s third-quarter report conjectured that their vaccine could be on the market by the third week of November if their Emergency Use Authorization is approved by the FDA.
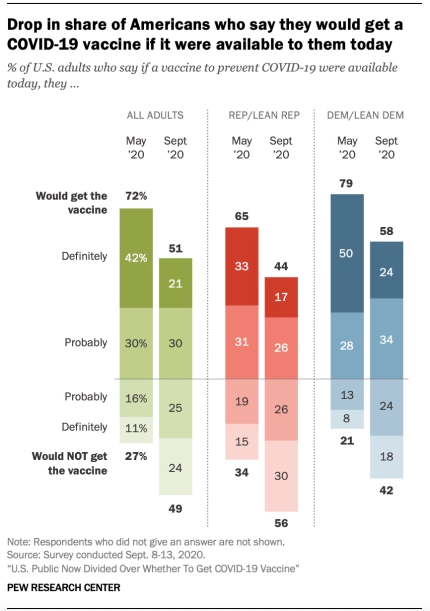
However, many Americans are skeptical about the safety of the vaccine. There are fears of the already evident neurological damage and other long-term health consequences. In a poll taken by Pew Research in September, 51% of adults polled said they would receive the vaccine. By contrast, in May, 72% of the adult population polled said they would receive the vaccine.
Senior Allison Santa-Cruz is also a skeptic.
“I would need more information on the vaccine before I got it,” said Santa-Cruz.
Russia and China have already given a vaccine to their citizens. There is much international skepticism concerning their rapid vaccine development. Many critics say they would not trust the Chinese and Russian vaccine if offered. Granted, the Russian vaccine was released before stage 3 trial approval.